Cambrex completes large new manufacturing addition
To The Press
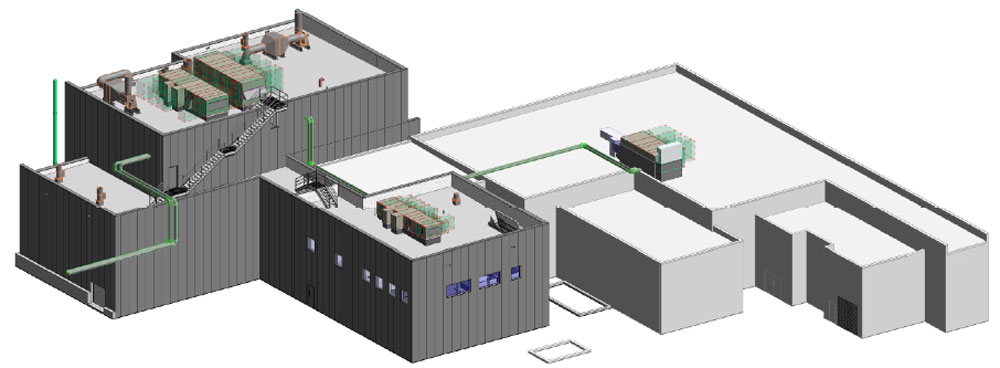
Cambrex Corp. has completed construction of a $24 million manufacturing facility at its site in Charles City, the company announced Wednesday.
The 6,000-square-foot addition is currently undergoing validation and will be ready to start customer projects next month, Cambrex said in a press statement.
The addition will manufacture highly potent active pharmaceutical ingredients (APIs).
“Across our sites, Cambrex has a strong reputation in the handling and supply of potent molecules, and this investment allows us to increase the capacity we can offer our customers,” said John Andrews, vice president of operations and site director in Charles City.
“We have seen an increased number of molecules in the clinical pipeline being designated as potent and highly potent, so having the flexibility within our manufacturing network to scale up with existing customers as projects progress, as well as accommodate new projects, is crucial to meet those market needs,” Andrews said.
The production area will operate to an occupational exposure limit down to one-tenth of a microgram per cubic meter. A microgram is one-millionth of a gram.
The area contains four reactor vessels, ranging from 200-gallon to 1,000-gallon capacity, enabling manufacturing batch sizes up to 300 kilograms, or about 660 pounds.
With the completion of this new facility, the Charles City site now has the flexibility to support all phases of development and offer all scales of highly potent active pharmaceutical ingredients manufacture across the full occupational exposure limit band spectrum, the company said.
Cambrex’s Charles City facility employs more than 370 people and is located on a 45-acre site in the industrial area in the southwest part of the city.
It is part of the company’s drug substance business unit and manufactures a wide range of APIs and pharmaceutical intermediates, including highly potent molecules and controlled substances.
Cambrex Corp. is the leading small molecule company, providing drug substance, drug product and analytical services across the entire drug lifecycle, the company said. It provides customers with an end-to-end partnership for research, development and manufacture of small molecule therapeutics.
Cambrex has 35 years’ experience and more than 2,000 experts servicing global clients from sites in North America and Europe.











Social Share